Calculations for Standard Cubical Unit Lattice
Calculations for Standard Cubical Unit Lattice: Overview
This topic covers concepts, such as, Density of a Cubic Crystal System, Coordination Number, Relation between Edge Length and Radius of a Constituting Atom for a Simple Cubic Structure & Effect of Temperature and Pressure on Coordination Number etc.
Important Questions on Calculations for Standard Cubical Unit Lattice
Silver crystallises with face-centred cubic unit cells and each side of the unit cell has a length of . Determine the radius of an atom of silver. (Assume that each face atom is touching the four corner atoms.)
Calcium crystallizes in a face centred cubic unit cell with a The density of the metal if it contains 0.1% schottky defects would be:
Copper crystallises in a face-centred cubic lattice and has a density of at The radius of a copper atom is:
[Atomic mass of
Iron has a body-centered cubic unit cell of cell edge . The density of iron is . The Avogadro number is
(Atomic mass of iron )
X-rays diffraction studies show that copper crystallizes in an FCC unit cell with cell edge of In a separate experiment, copper is determined to have a density of , the atomic mass of copper would be:
In face-centred cubic and body centred cubic whose unit cell lengths are and respectively, a metal crystallises into two cubic phases. What is the ratio of densities of and
A unit cell of sodium chloride has four formula units with an edge length of the unit cell . What is the density of sodium chloride?
A metallic element crystallises into lattice having a layering sequence of Any packing of sphere leaves out voids in the lattice. Determine what percentage by volume of this lattice is empty space.
The coordination number of a metal crystallizing in a hexagonal close-packed structure is:
has bcc structure with edge length . The shortest inter ionic distance in between and is:
Atoms of metals and form face-centred cubic (fcc) unit cell of edge length , body-centred cubic (bcc) unit cell of edge length , and simple cubic unit cell of edge length , respectively. If and , then the correct statement(s) is(are)
[Given: , and are molar masses of metals , and , respectively. , and are atomic radii of metals , and , respectively.]
Atom occupies the fcc lattice sites as well as alternate tetrahedral voids of the same lattice. The packing efficiency (in ) of the resultant solid is closest to
Copper crystallizes in an FCC unit cell with cell edge of The density of copper is , Calculate the atomic mass of copper.
Ice crystallises in a hexagonal lattice. At the low temperature at which the structure was determined, the lattice constants were and . How many molecules are contained in a unit cell? The density of ice is at A unit cell of is shown below:
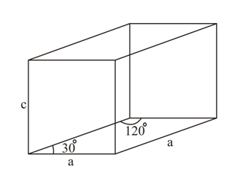
What is the radius of sodium atom if it crystallizes in structure with the cell edge of ?
A unit cell of sodium chloride has four formula units. The edge of length of the unit cell is What is the density of sodium chloride. Atomic Mass of
(atomic weight ) crystallizes in the cubic system with . Its density is . Determine the cell type. Calculate the radius of atom.
If the radius of a metal atom is and its crystal structure in cubic close packed ( lattice), what is the volume (in ) of one unit cell?
Copper crystallises in a structure of face centerd cubic unit cell. The atomic radius of copper is . What is axial length on an edge of copper.
Zinc selenide crystallizes in a face-centred cubic unit cell and has a density of Calculate the edge length of the unit cell.
Give the answer as the nearest integer.
